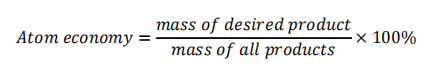Green chemistry
appreciate that green chemistry principles include the design of chemical
synthesis processes that use renewable raw materials, limit the use of
potentially harmful solvents and minimise the amount of unwanted products
- Purpose of green chemistry
- Aims to maximise the desired products and minimise by-products thereby improving the atom economy of a process. It is desirable to design and produce economically viable chemical products and devise processes that reduce pollution at its source.
- 12 green principles
- Prevent waste
- Maximise atom economy
- Design less hazardous chemical synthesis
- Design safer chemicals and products
- Use safer solvents
- Increase energy efficiency
- Use renewable raw materials
- Avoid chemical derivatives
- Use catalysts
- Design for degradation
- Real-time pollution prevention
- Minimise potential for accidents
- Key definitions
- Biodegradable - materials that are broken down by microorganisms in soil
- Renewable - can be replaced at a rate faster or equal to the rate at which it is being consumed
- Bioaccumulation - when chemicals build up in the tissues of organisms, typically at the top of the food chain
outline the principles of green chemistry and recognise that the higher the atom economy, the 'greener' the process
Waste prevention
- Design a process to reduce the amount of waste, however waste products can either be:
- Used for another purpose
- Biodegradable
Atom economy
- Atom economy is a measure of how much reactant is converted to usable products
- Combination reactions will always be considered 'greener' processes due to only having one product
- 100% atom economy means all atoms in the reactants are being converted to desired product
- Maximising atom economy will minimise waste



calculate atom economy and draw conclusions about the economic and environmental impact of chemical synthesis processes.