Organic materials
Proteins
appreciate that organic materials including proteins, carbohydrates, lipids
and synthetic polymers display properties including strength, density and
biodegradability that can be explained by considering the primary, secondary
and tertiary structures of the materials
describe and explain the primary, secondary (a-helix and B-pleated sheets),
tertiary and quaternary structure of proteins
recognise that enzymes are proteins and describe the characteristics of
biological catalysts (enzymes) including that activity depends on the structure
and the specificity of the enzyme action
- The monomer of a protein is an amino acid
▪ Non-polar (alanine)
▪ Polar (serine)
▪ Proton donors (aspartic acid)
▪ Proton acceptors (lysine)
▪ -NH2 group can act as base (accepting proton to become a -NH3+ group)
▪ -COOH group can act as an acid (donating proton to become a -COO- group)
▪ Amino acid present in cationic form in solutions of low pH and anionic form in solutions of high pH
▪ Reversible reaction - in aqueous solution of amino acid, there is equilibrium mixture amino acid molecules and zwitterions
Primary structure
- Primary structure of protein is the sequence of amino acids, joined by peptide bonds
▪ Function of proteins is highly dependent on the order of amino acids

Explanation: why do proteins fold into 3D structures?
- Two main reasons - minimise repulsion, maximise attraction between amino acids and surroundings
Tertiary structure
- Tertiary structure is the 3D interactions between numerous secondary structures present in a polypeptide
▪ Non-polar side chains - dispersion forces (hydrophobic)
▪ Polar - dipole-dipole or H-boning (hydrophilic)
▪ Ionic - electrostatic interactions (soluble)
▪ Disulfide bridge - covalent bonding (between nearby sulfur containing side chains
Quaternary structure
- Quaternary structure is the folding of multiple protein chains together
Enzymatic catalysis
- Enzymes are a biological catalyst, which reduce the activation energy for a reaction
- Differences between inorganic catalysts and enzymes
▪ Lose catalytic activity outside pH and temperature range
▪ This is because proteins 3D structure is affected by pH and temperature
What does "all folding is disrupted by excessive heat
and either basic or acidic conditions" mean?
When you increase the temperature, the kinetic
energy will increase. Excessive vibrations will
eventually breakdown the IMF’s holing the protein
together. The weakest will break first. It is the IMF’s
that are holding the molecule in a particular shape
allowing it to work as a catalyst. As the pH changes,
the NH group can act as a base, along with the side
chains that can accept or donate a proton. This
changes/disrupts the existing IMF’s therefore
denaturing the protein.
Carbohydrates
appreciate that organic materials including proteins, carbohydrates, lipids and synthetic polymers display properties including strength, density and biodegradability that can be explained by considering the primary, secondary and tertiary structures of the materials
recognise that monosaccharides contain either an aldehyde group (aldose) or a ketone group (ketose) and several -OH groups, and have the empirical formula CH 0
distinguish between a-glucose and B-glucose, and compare and explain the structural properties of starch (amylose and amylopectin) and cellulose
Carbohydrates are natural occurring sugars and their polymers
- They are polyhydroxy aldehydes or ketones, and the molecules they form through condensation
- Monosaccharides
Monosaccharides are single sugar monomers
Disaccharides
Polysaccharides
Liquids
appreciate that organic materials including proteins, carbohydrates, lipids
and synthetic polymers display properties including strength, density and
biodegradability that can be explained by considering the primary, secondary
and tertiary structures of the materials
recognise that triglycerides (lipids) are esters and describe the difference in
structure between saturated and unsaturated fatty acids
describe, using equations, the base hydrolysis (saponification) of fats
(triglycerides) to produce glycerol and its long chain fatty acid salt (soap),
and explain how their cleaning action and solubility in hard water is related to
their chemical structure
A type of lipid is triglycerides
- Triglyceride is made from glycerol (alcohol) and 3 fatty acids
- Condensation reaction
Explanation: why are saturated fats bad for the body, compared to unsaturated fats?
- Saturated fats are very stable and do not easily react with other molecules
▪ Acidic group at end of fatty acid is reactive, and allows for oxidation of the chain by cutting it to pieces, two carbons at a time
- An unsaturated fat is more chemically active, and is much more readily oxidised by the body
Soaps
- Saponification (or base hydrolysis) is the organic reaction between an acidic ester and a strong base to produce a fatty acid salt

- Soaps are amphipathic - have both hydrophobic and hydrophilic parts

- Cleaning action
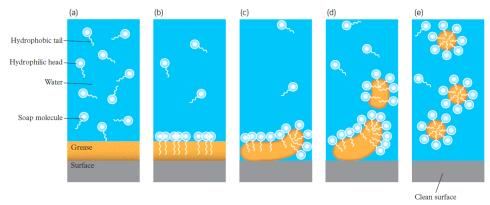
- Micelles
▪ Pick up dirt by forming around the grease droplets, allowing them to form an emulsion in water
▪ Micelles repel other micelles. Allowing them to be stable.
Hard water
- In hard water (containing dissolved Ca+2 and Mg+2) - sodium stearate (soap) is soluble but calcium and magnesium are not and precipitate as scum
explain how the properties of polymers depends on their structural features
including; the degree of branching in polyethene (LDPE and HDPE), the
position of the methyl group in polypropene (syntactic, isotactic and atactic)
and polytetrafluorethene.