Structure of organic compounds
recognise that organic molecules have a hydrocarbon skeleton and can contain functional groups, including alkenes, alcohols, aldehydes, ketones, carboxylic acids, haloalkanes, esters, nitriles, amines, amides and that structural formulas (condensed and extended) can be used to show the arrangement of atoms and bonding in organic molecules
- Hydrocarbons are an organic compound consisting of only carbon and hydrogen atoms
- Functional groups are an atom or a group of atoms in a organic compound that determines the reactivity of that compound.
Order of priority functional groups in polyfunctional compounds
- Homologous series is a class of molecules in which each member differs by -CH2- from the previous member
▪ A similar structure
▪ A pattern to physical properties
▪ Similar chemical properties
▪ Same general formula
- Comparing methods of notating a chemical
deduce the structural formulas and apply IUPAC rules in the nomenclature of organic compounds (parent chain up to 10 carbon atoms) with simple branching for alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, haloalkanes, esters, nitriles, amines and amides
- Conventions when naming hydrocarbons
▪ Longest chain must include the double or triple bond for alkenes and alkynes
▪ Stem must contain functional group and function group takes priority numbering
- Cyclic compounds
- Resonance structures
Benzene is a cyclic compound (C6H6)
Electrons in double bond are not fixed in position. They are delocalised and can move around - forming resonance
▪ Benzene is more stable (and less reactive) than most unsaturated molecules because its delocalised electrons are not as available to take part in reactions
identify structural isomers as compounds with the same molecular formula but different arrangement of atoms; deduce the structural formulas and apply IUPAC rules in the nomenclature for isomers of the non-cyclic alkanes up to Св
- Structural isomers are two or more organic compounds that have the same atoms but a different arrangement and bonding
Chain isomers are consequence of the branching possible in carbon chains
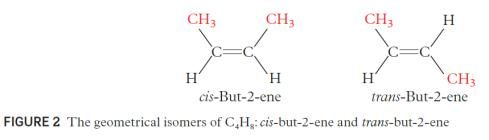
identify stereoisomers as compounds with the same structural formula but with different arrangement of atoms in space; describe and explain geometrical (cis and trans) isomerism in non-cyclic alkenes.
- Stereoisomers are two or more organic compounds that have the same atoms and bonding but a different spatial arrangement
○ Geometrical isomers are two organic compounds that have different arrangements of atoms around a rigid double bond